- EUR-LIVE
- eur-live@u-pec.fr
Publié le 20 juin 2025
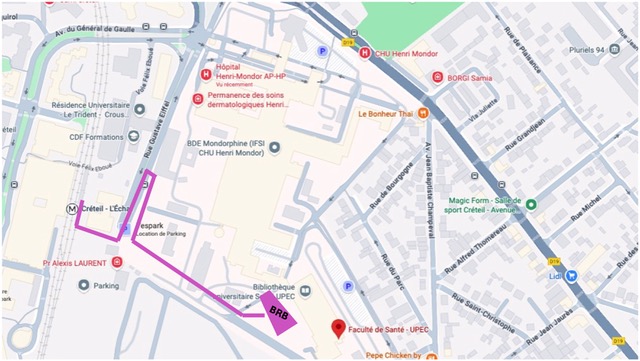
L'évènement auras lieu au bâtiment de recherche biomédicale, faculté de santé UPEC, 8 rue du général Sarrail 94000 Créteil.
Inscription obligatoire : https://forms.cloud.microsoft/e/ztV1DS8GPB
Adresse : Bâtiment de Recherche Biomédicale BRB, Faculté de Santé UPEC
8 rue du général Sarrail 94000 Créteil
Nous sommes ravis de vous annoncer la Summer School 2025, un événement entièrement conçu dans la continuité de nos précédents événements axés sur la question du vieillissement. Cette année, l'événement s'intitule "Sénescence et vieillissement : des mécanismes moléculaires aux interventions multi-échelles".
Rejoignez des experts de renom et de jeunes talents dans le domaine de la recherche sur le vieillissement et la sénescence lors de cette conférence révolutionnaire de deux jours. Elle réunira les dernières découvertes, les technologies innovantes et les avancées thérapeutiques pour décrypter la biologie complexe du vieillissement et de la sénescence. Que vous soyez spécialisé en biologie moléculaire, en modélisation computationnelle, en recherche clinique ou en santé publique, cette conférence offre une plateforme interdisciplinaire unique pour explorer le vieillissement, de la cellule à la société.
La Summer School 2025 est créer par l'EUR LIVE, en collaboration avec des universités européennes de renom telles que l'Université de Glasgow, l'Université Federico II de Naples et l'alliance universitaire européenne Aurora.
Nous vous attendons nombreux pour ces deux jours de conférences et de discussions.
Pourquoi participer ?
• Science de pointe : Plongez au cœur des mécanismes moléculaires et cellulaires à l’origine de la sénescence et du vieillissement, notamment les horloges épigénétiques, la dynamique mitochondriale et les interactions avec le système immunitaire.
• Technologies innovantes : Découvrez comment la multiomique spatiale, l’analyse des big data et l’édition épigénétique de précision révolutionnent la recherche sur le vieillissement.
• Perspectives translationnelles : Explorez les stratégies thérapeutiques ciblant les cellules sénescentes, telles que la co-ciblage sénolytique dans le cancer et le vieillissement cardiaque, ainsi que les approches d’élimination médiée par le système immunitaire.
• Approches systémiques et de santé publique : Comprenez comment les modèles intégratifs et les politiques publiques peuvent orienter des interventions visant à ralentir le vieillissement au niveau de l’organisme et des populations.
• Réseautage et collaboration : Connectez-vous avec des scientifiques de renom, des cliniciens et des décideurs. Participez à des tables rondes et des panels consacrés à l’avenir de la recherche sur le vieillissement.
• À la découverte des talents émergents : Rencontrez les leaders de demain lors de sessions « flash talk » mettant en lumière les recherches novatrices de jeunes chercheurs.
Sessions phares :
• La sénescence cellulaire comme moteur systémique du vieillissement et des maladies
• Plateformes de découverte multi-échelles : des produits naturels à l’omique spatiale
• Quantifier et reprogrammer le vieillissement : big data et horloges épigénétiques
• Ciblage thérapeutique de la sénescence : interfaces organiques et surveillance immunitaire
• Du vieillissement cellulaire à la santé publique : approches intégratives pour ralentir la sénescence
Conférencier d’honneur :
Clemens Schmitt — Pionnier des sénothérapies ciblant simultanément le cancer et le vieillissement.
À qui s’adresse cet événement ?
Aux chercheurs en biologie moléculaire, génétique, épigénétique, immunologie, bioinformatique, pharmacologie, sciences cliniques et santé publique, ainsi qu’aux innovateurs en biotechnologie et aux décideurs intéressés par le vieillissement et les maladies liées à l’âge.
Inscription obligatoire : https://forms.cloud.microsoft/e/ztV1DS8GPB
Les participants pourront se joindre à l'événement en ligne. Vous recevrez un lien pour y accéder après inscription.
10h00 - 10h30 : Pause-café
12h30 - 14h00 : Pause déjeuner et session d'affichage des posters I
15h15 - 16h00 : Pause café
10h00 - 10h45 : Pause-café
12h30 - 14h00 : Pause déjeuner et session d'affichage des posters II
15h45 - 16h00 : Remarques de clôture et points clés à retenir
Inscription obligatoire : https://forms.cloud.microsoft/e/ztV1DS8GPB
Mael Lemoine:
Contemporary biogerontology is largely driven by "aging cell reductionism": the hypothesis that intracellular degradations primarily explain other aspects of the aging of organisms (typically, Lopez-Otin 2013). Indeed, "cell-autonomous" processes are not caused by extrinsic influence of the tissue (e.g., by concentration of byproducts of other cells, alteration of the extracellular matrix, etc.). The question is whether they really cause all aspects of aging. Experimental research programs generally presuppose it and investigate cell-autonomous processes or admit that non-cell-autonomous processes are derived from them. I want to develop 3 arguments to establish that the study of "cell-autonomous" processes of aging should be interpreted in the light of a cell-population theory, that cumulated perturbations are independent from cell damage, and that many variations in the rate of aging are not likely to come from variations in cell aging.
Utz Hebig:
Although senescent cells (SCs) perform important functions in various biological processes, their timely elimination by immune cells aner they are generated is critical to maintain tissue homeostasis, suppress aging, and prevent disease. The observations that SCs accumulate in tissue with advancing age, however, suggest that at least some develop mechanisms that prevent their elimination, or that immune cells capable of targeting SCs also increasingly become dysfunctional with age. I will discuss the role of immune cells in eliminating senescent cells (SCs) systemically and present evidence linking senescent and dysfunctional T cells to the promotion of age-associated diseases and disorders in humans.
Clemens A. Schmitt:
Cellular senescence underlies most, if not all, age-related pathologies – among them organ fibrosis,cardiovascular diseases, neurodegeneration, and cancer – as a central component of increasingly futile or improper tissue regeneration, leading to miscoordinated inflammation, immune interference, extracellular matrix remodeling, and stemness. While serving as an important barrier to full-blown tumor formation, lastingly remaining (‘uncleared’) senescent (pre)cancer cells, especially treatment- surviving ‘persisters’, bear the risk of re-entering the cell-cycle and driving particularly aggressive relapses. Hence, targeting manifest senescent cancer cells in a consolidative manner, eliminating senescent precursor lesions or even preventing their potential progression from an indiscernible state, may profoundly impact the incidence and outcome of cancer. At the same time, senolytic targeting could unleash significant anti-aging effects beyond cancer control and prevention.
Alex Chang:
Both doxorubicin and ischemic reperfusion (I/R) are known drivers of heart failure clinically, but the ageof onset differs. Current dogma states that the cardiotoxicity of doxorubicin is dose-dependent, yet the clinical manifestations in childhood-exposure-adult-onset cancer survivors remain variable. Ischemic reperfusion damage causes rapid mitochondrial fragmentation in cardiomyocytes, and despite vascular reperfusion surgeries, contractile function continues to deteriorate, marked by mitochondrial dysfunction. To study whether mitochondrial amount confers resistance against myocardial insult, we established isogenic hiPSC-CMs containing high, normal, and low mitochondrial copy numbers using mitochondrial transplantation (mito high) and the YFP-Parkin +CCCP (mito low) system. Mechanistically, both doxorubicin and I/R challenges lead to mPTP opening and mitochondrial DNA (mtDNA) leakage. mtDNA leakage activates the cGAS-STING pathway, driving inflammation and myocardial senescence. Prolonged cGAS/STING activation also results in MFN2 recruitment, leading to mitochondrial hyperfusion. Cardiomyocyte-specific knockout of Sting (Myh6-Cre x Sting flox/flox; Sting CKO) and overexpression of mitochondrial tagged DNase1 (mtDNAse1) in mice rescue mitochondrial defects and restore cardiac function. Together, we provide evidence that mitochondrial content determines myocardial durability and that mtDNA leakage can drive myocardial senescence in two pathological sejngs.
Jesus Gil:
Senescent cells are induced in response to oncogenic activation and tissue damage, and the immune system typically removes them. When the immune surveillance of senescent cells is ineffective, these cells persist, as observed in aged, cancerous, and fibrotic tissues. The abnormal accumulation of senescent cells is linked to cancer and various age-related diseases. Recently, drugs that selectively eliminate senescent cells, known as senolytics, have shown promise in improving outcomes for many of these conditions. An alternative method to clear senescent cells involves enhancing their immune-mediated clearance. I will describe our screenings to identify strategies for improving the elimination of senescent cells by Natural Killer (NK) cells. We find that SMARCA4 regulates the secretion of immunomodulatory chemokines by senescent cells. A PROTAC targeting SMARCA4 increases the senescence-associated secretory phenotype (SASP), enhances NK-mediated killing of senescent cells, and synergizes with cisplatin to promote the infiltration of CD8 T cells and mature, activated NK cells in an immunocompetent model of ovarian cancer. Our results indicate that SMARCA4 inhibitors improve NK-mediated surveillance of senescent cells and may provide senotherapeutic options for ovarian cancer and other senescence-associated diseases.
Christoph Kuppe:
Understanding the complex cellular ecosystems of the human heart in health and disease requires an integrative approach to multi-modal data. In this talk, I present a novel multimodal graph optimal transport (GraphOT) framework designed to perform patient-level multiomics integration, focusing on human myocardial infarction (MI). By combining high-throughput single-nucleus RNA sequencing (snRNA-seq) with spatial transcriptomics from FFPE tissue, we build detailed atlases of cellular heterogeneity and spatial organization in the diseased heart. We uncover distinct cardiomyocyte (CM) states—including novel, disease-associated polyploid populations—characterized by altered sarcomeric gene expression and alternative RNA splicing, both of which are enriched in heart failure contexts.Integration of GWAS-derived single-cell disease relevance scores strengthens the clinical link of these CM states, highlighting their potential as therapeutic targets. Furthermore, we explore polyploid cardiomyocyte-specific expression signatures and splicing regulators, suggesting functional roles in heart failure pathology. The PiLOT trajectory framework, incorporating histology and transcriptomics, enables temporal mapping of myocardial remodeling across species, further supported by comparative analyses with mouse models. Our collaborative, multi-center effort outlines a translational systems biology approach to decode human cardiac disease and guide precision therapy development.
Wolfgang Wagner:
Aging is reflected in genome-wide DNA methylation changes, which form the basis of epigenetic clocks.However, it remains largely unclear how these epigenetic modifications are regulated and whether they directly affect the aging process. I will present new approaches for epigenetic aging signatures based on weighted 2D kernel density estimation, which may also provide a novel measure of biological age. Furthermore, we performed epigenetic editing at age-associated CpG sites to explore the consequences of interfering with epigenetic clocks. CRISPR-guided editing targeted individual age-related CpGs, evoking genome-wide bystander effects that were highly reproducible and enriched in other age-associated regions. 4C-sequencing at age-associated sites revealed increased interactions with bystander modifications and other age-related CpGs. Subsequently, we multiplexed epigenetic editing in human T cells and mesenchymal stromal cells at five genomic regions that become either hypermethylated or hypomethylated with aging. While targeted methylation appeared more stable at age-hypermethylated sites, both approaches induced bystander modifications at CpGs with the highest correlations to chronological age. Notably, these effects were simultaneously observed at CpGs that both gain and lose methylation with age. These results demonstrate that epigenetic editing can extensively modulate the epigenetic aging network and interfere with epigenetic clocks. Furthermore, I will provide an update on ongoing analyses integrating age-associated genome-wide DNA methylation changes with histone modifications and chromatin accessibility.
Isabelle ADER-PERARNAU:
Aging is a process marked by a decline in functions and is a main risk for age-related diseases, impacting health and survival. The WHO monitors five key domains (cognition, locomotion, sensory, vitality, psychological) to assess health, as frailty signals unhealthy aging and risk of dependency. Intrinsic Capacity (IC), combining these domains, predicts overall physical and mental abilities, aiding in evaluating health, comorbidity, and frailty. A key challenge is linking molecular markers to IC, which could enhance understanding of functional decline and guide early interventions. Our multiscale study aims to identify molecular markers of IC using samples from the INSPIRE cohort, which includes 1,000 plasma and 133 skin fibroblast participants aged 20-96, with varying frailty and IC scores. Plasma analysis explored energy metabolism related to functional decline, while dermal fibroblasts revealed aging and IC mechanisms.
Jing Ye:
Heart failure (HF) is a significant public health concern, with its prevalence increasing as the population ages. However, the molecular etiologies of HF remain largely unknown. Here, we show that the downregulation of cardiomyocyte telomeric repeat-binding factor 2 (TRF2) in cardiomyocytes, observed during normal aging, serves as a breeding ground for heart failure. Mechanistically, we reveal a noncanonical role of TRF2 in cardiomyocyte mitochondria, preventing oxidative stress, which is impaired by a twofold reduction in TRF2 levels—a mechanism sufficient to trigger an HF phenotype. This study provides insights into HF pathogenesis by revealing that the age-related downregulation of the telomere factor TRF2 in cardiomyocytes is causal to HF. This offers guidance for the development of novel prevention and therapeutic strategies. Moreover, it unveils how a molecular change occurring during normal aging can serve as a breeding ground for a pathological outcome in the case of an adverse event.
Laurent MEIJER:
Memory and learning disorders observed in patients with Down syndrome (DS) and Alzheimer’s disease (AD) are associated with an excess activity of the DYRK1A protein kinase. Genetic and pharmacological inhibition of DYRK1A corrects these cognitive disorders in DS/AD animal models, encouraging the development of a clinical DYRK1A inhibitor to treat memory/learning difficulties in patients with AD or DS. Inspired by a marine sponge natural product, Leucegamine B, we synthesized, optimized, and extensively characterized over 1200 analogues, Leucejnes [1-3] and Leucejnibs [4, 5]. We also selected an orally available drug candidate, Leucejnib-21 [6, 7], which was formulated in immediate-release tablets. A double-blind, placebo-controlled clinical phase 1 study (‘Leucega’) involving 120 participants was launched in January 2024 to investigate the safety and pharmacokinetics of Leucejnib-21 [8]. The Leucega trial comprises Single Ascending Dose, Food Effect, and Mul@ple Ascending Dose studies in 96 healthy volunteers. In addition, 12 adults with DS and 12 AD patients will receive a single Leucejnib-21 dose for pharmacokinetics and biomarkers analysis. Blood plasma samples collected from all participants will be used to analyze plasma proteomic and phosphoproteomic profiles, as well as AD-specific biomarkers, especially pThr212-Tau (a phosphorylation site for DYRK1A and recently reported DS/AD biomarker [9, 10]) and p-Thr217-Tau. The expression level and activity of DYRK1A will be evaluated in plasma and PBMCs. The Leucega study will be completed by the end of Q2-2025, and the results of this phase 1 trial will be presented. In addition, we will present and discuss the planned phase 2A protocol (not finalized at this stage, but double-blind and placebo-controlled), which will address safety, measure the above-mentioned plasma biomarkers, and make use of various methods to evaluate the ability of Leucejnib-21 to correct cognitive disorders in children with DS. If funds allow, other therapeutic indications where DYRK1A also appears to be involved, such as Parkinson’s disease, dementia with Lewy bodies, myocardial infarction, and type 2 diabetes, will be investigated.
Didier Coeurnelle:
Life expectancy is rising again after the COVID-19 pandemic. Addressing aging-related diseases presents a growing challenge to healthcare systems and research institutions. Advances in artificial intelligence (AI), combined with large-scale health datasets—ranging from genomics and longitudinal patient records to environmental and lifestyle data—offer unprecedented opportunities to uncover biological mechanisms of senescence and identify interventions to extend healthy lifespan. However, the current landscape is fragmented, with most progress driven by private actors and limited access to data for the majority of scientists, mainly and paradoxically, those working for public institutions. This intervention argues that public institutions have the capacity and the ethical imperative to lead coordinated efforts using AI to analyze big health data for insights into biological aging. We examine technical methodologies (e.g., deep learning, causal inference models, federated learning), biological targets, current data gaps, and legal limitations. We propose open-access research infrastructures (with data anonymization or pseudonymization). Case studies from existing national biobanks and European digital health initiatives illustrate opportunities and constraints. We conclude by outlining a roadmap for public engagement, potentially at the EU level, which includes the need for an AI-literate and altruistic publichealth scientific community to promote healthy longevity. Public institutions can play a transformative role in slowing the aging-senescence process for all.
Julien Cherfils:
The accumulation of senescent cells (SCs) in tissues is now recognized as a key driver of aging and age- related diseases. While some SCs are eliminated by the immune system, others persist and accumulate over time, suggesting the existence of mechanisms regulating their immunogenicity. In our recent work (Iltis et al., Nature Aging, 2024), we demonstrated that SCs induced by various non-oncogenic stresses evade natural killer (NK) cell surveillance through a profound remodeling of their glycocalyx, notably characterized by a marked increase in the disialylated ganglioside GD3. We found that high GD3 expression, observed in organs such as the liver, lung, kidney, and bone during aging, generates a strong immunosuppressive signal that limits the immune clearance of SCs. These GD3+ SCs contribute to the development of age-related diseases and the aging phenotype. Therapeutic targeting of GD3 with anti-GD3 monoclonal antibodies in aged mice restores immune surveillance and significantly agenuates age-associated pathologies, including fibrosis in multiple organs. Importantly, anti-GD3 treatment not only reduces fibrosis but also increases healthspan, the period of life spent in good health. Furthermore, our recent findings reveal a significant impact of sexual dimorphism on the efficacy of anti-GD3 therapy, emphasizing the need for sex-specific approaches in senescence-targeting strategies. In conclusion, GD3 emerges as a genuine “senescence immune checkpoint” that determines the fate of senescent cells and represents a promising therapeutic target for improving health and longevity.
Stefanie Dimmler:
Aging is a major risk factor for impaired cardiovascular health, yet the cellular mechanisms underlying age-related cardiac dysfunction remain incompletely understood. Given the close anatomical and functional relationship between nerves and blood vessels, we investigated the impact of aging on the cardiac neurovascular interface. We found that aging leads to reduced ventricular nerve density and dysregulation of vascular-derived neuroregulatory signals, including downregulation of microRNA-145 (miR-145) and subsequent derepression of the neurorepulsive factor semaphorin-3A (Sema3a). Notably, deletion of miR-145 or endothelial overexpression of Sema3a recapitulated the aged-heart phenotype by reducing axon density. Importantly, we identified senescent cells as key contributors to this process: their accumulation correlated with denervation, and their targeted removal reversed Sema3a expression, restored nerve density, improved cardiac autonomic regulation, and reduced electrical instability. These findings highlight cellular senescence as a critical mediator of neurovascular dysfunction in the aging heart and a potential therapeutic target for preserving cardiovascular health in the elderly.
Pasquale Maffia:
Chronic inflammation and immune dysregulation are now recognised as key contributors to the development and progression of cardiovascular diseases, including atherosclerosis, hypertension, and heart failure. This talk will explore recent advances in understanding the immune mechanisms driving these conditions, with a focus on how immune cells, particularly antigen-presenting cells and T lymphocytes, orchestrate pathological responses in the vasculature and the heart. Drawing from cutting-edge imaging techniques and translational studies, I will highlight how immune cell interactions shape cardiovascular pathology, and discuss emerging insights into the role of infection and inflammation in global cardiovascular health. Special attention will be given to how these mechanisms intersect with aging and senescence, offering novel perspectives for therapeutic targeting in age-associated cardiovascular disease.
Vincent GELI:
We developed two genetically engineered mouse models—p21⁺/Tert and p21⁺/TertCi—in which the telomerase reverse transcriptase (Tert) or its catalytically inactive version (Tert^Ci) is expressed under the control of the p21^Cdkn1a promoter, a key regulator of cellular senescence. This design aimed to establish a feedback loop that enables conditional TERT expression in pre-senescent cells, thereby reducing senescence-associated inflammation and tissue dysfunction. Our results demonstrate that TERT expression under p21 control confers anti-senescence, antioxidant, and anti-inflammatory benefits, supporting the maintenance of stem and progenitor cells across multiple tissues, including those under metabolic stress, such as obesity. These effects involved both canonical telomerase activity and non-canonical TERT functions. Notably, between 17 and 20 months of age, 15% of these mice developed liver tumors histologically similar to human hepatocellular carcinoma (HCC). Whole-exome and transcriptome analyses of nine tumors revealed distinct mutation patterns: p21⁺/Tert tumors frequently harbored activating Ctnnb1 mutations, whereas p21⁺/TertCi tumors showed alterations in Hras or Ep300. RNA-seq profiling revealed the activation of Wnt/β-catenin, Erk, and Notch signaling pathways, which correlated with their mutational landscape. Tumors also differed in their resilience to oxidative stress and in ferroptosis-related gene expression. Mechanisms of senescence bypass involved metabolic rewiring and aberrant Hnf4a expression. Notably, Ctnnb1-mutant tumors exhibited reduced immune cell infiltration compared to Hras-mutant tumors. Together, these findings underscore the multifaceted roles of TERT in modulating aging, cellular stress responses, and tumorigenesis.

Mise à jour le 31 oct. 2023